Written by: Eline te Braake
In recent years, a lot of useful and promising eHealth technologies are developed. However, it is unfortunately still the case that a lot of these technologies are not (successfully) implemented in practice. It appears that daily practice is often very different than the research context. Furthermore, when in use, technology affects the behaviour of people, and vice versa, how people behave and use the technology, affects it’s impact. This can create a gap between the actual daily practice and the purpose of the eHealth technology. As a consequence, several eHealth technologies are only partial used or not at all used in practice. This means that potential end-users will never experience the benefits of these promising eHealth technologies.
How can we prevent this?
At RRD, we want to prevent this problem by looking at the implementation process from an early stage. Meaning that during the development of the eHealth technology, several steps are taken to decrease the chances of implementation failure. One of these steps is service modelling. A service model describes all the tasks, processes and responsibilities that certain people or organisations have or need to perform once the eHealth intervention will be put in daily practice. An important aspect of service modelling is to involve stakeholders; the people or organisations who affect or are affected by the technology. Stakeholders are the experts when it comes to daily practice, they have crucial knowledge about current struggles and strengths in practice which cannot be identified by solely looking at literature. Furthermore, by involving stakeholders, needs and wishes can be aligned which in time may increase the commitment towards future implementation.
Example of service modelling in a project
Recently, RRD developed the service model within the RE-SAMPLE project (Horizon grant no 965315). RE-SAMPLE is a European project that focuses on people with COPD. The goal of RE-SAMPLE is to develop a technology that supports patients and caregivers to manage their COPD and other chronic conditions. The RE-SAMPLE service model is based on 5 rounds of studies with stakeholders from three different countries: Italy, The Netherlands and Estonia.
Although RE-SAMPLE is one project, most of the studies were done for each country separately. It was very important to do this, because there are a lot of differences between the countries in terms of how care is organized. In Italy for example, it became clear that the physician spends most time with the patients, while in the Netherlands, pulmonary nurses see the patient with COPD more often. In the Netherlands, short waiting lists are seen as a strength of current care, while in Estonia, long waiting lists are mentioned as the weakness of current care. These are just small examples of the many differences between the three countries
Taking the differences into consideration, it appeared that one version of the service model cannot be used for all the three pilot sites. This is the reason that differences between countries are made clear in the service model. It might be that different roles and responsibilities are assigned to different stakeholders in a particular country. You can see the final version of the RE-SAMPLE service model with all the different roles, processes, and responsibilities below:
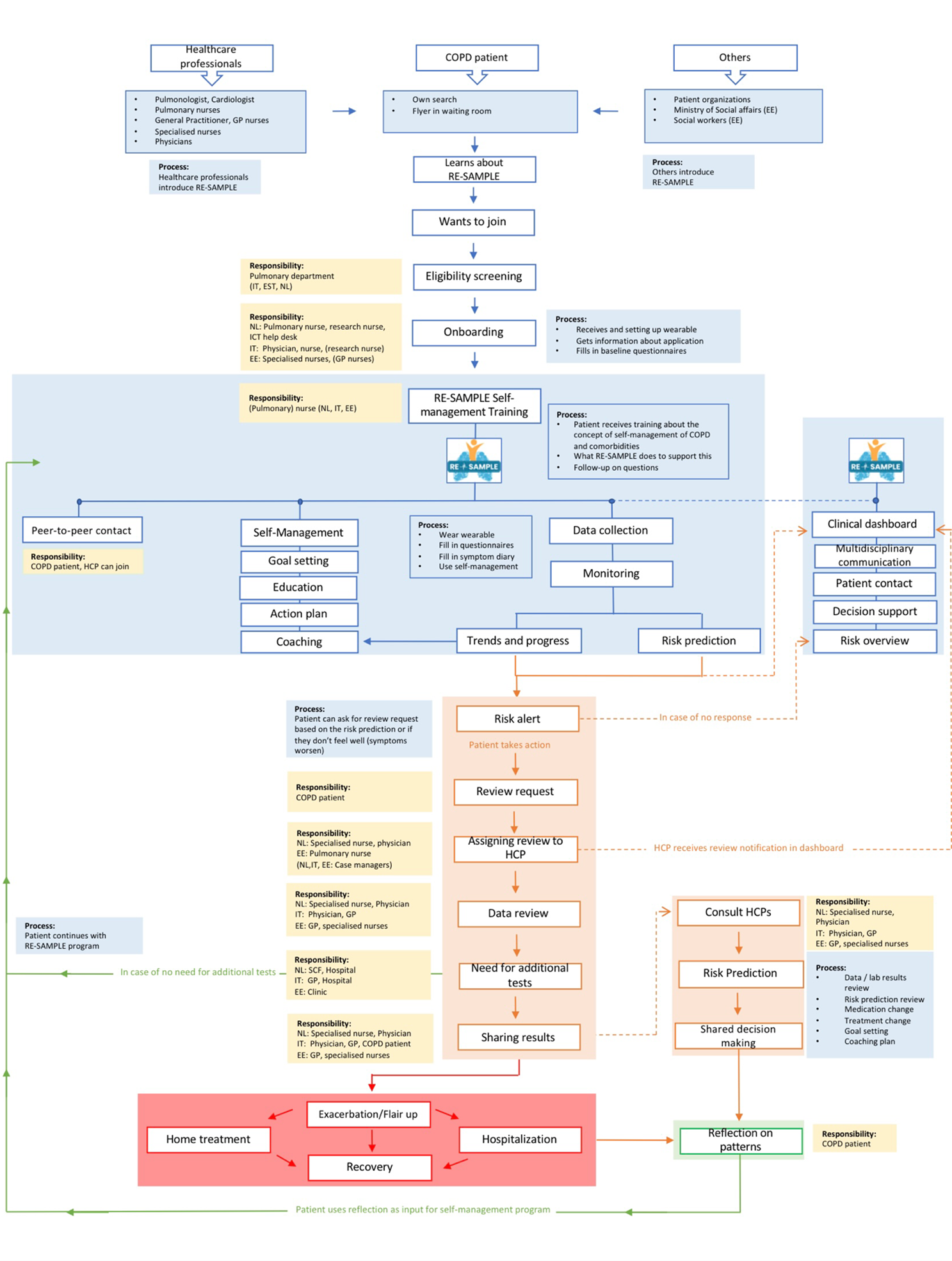
During the various surveys, much valuable information was gathered to develop the service model. Without stakeholder involvement, current processes, problems in care and preferences regarding future implementation could not be identified. Want to learn more about RE-SAMPLE's service model development? You can see a video below that explains the entire process of service model development in detail.
Would you like to see this process explained in Dutch? Then click on the following link: https://www.youtube.com/watch?v=S-PkshYyHMI
Do you want to learn more about service modelling in general or do you want to know what the options are for your organisation? Feel free to contact RRD! We can potentially help each other out and offer the help you need.